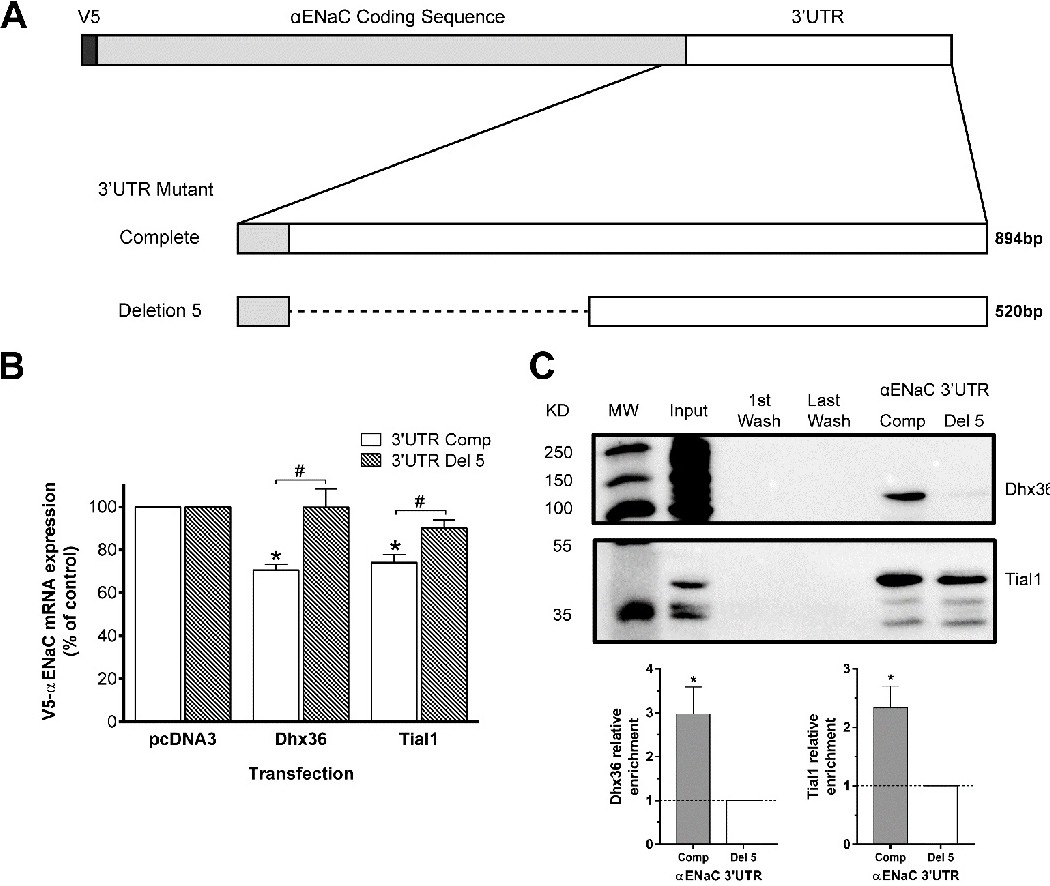
Fig. 7. Impact of the proximal region of the αENaC 3'UTR on the posttranscriptional modulation of V5-αENaC mRNA in cells that overexpress Dhx36 and Tial1. (A) The proximal portion of the αENaC 3'UTR was deleted by cloning the distal region of the 3'UTR next to the αENaC stop codon in the pTRE-tight plasmid (V5-αENaC-Del5). (B) Alveolar epithelial cells were cotransfected with V5-αENaC or V5-αENaC-Del5 in the pTRE-tight vector along with pTet-Off plasmid and the expression vector for Dhx36 or Tial1 RBP overexpression. V5-αENaC mRNA expression was quantified by RT-qPCR 72 h posttransfection and expressed as percentage ± SEM of V5-αENaC mRNA compared to cells transfected with an empty vector (pcDNA3) after normalization with tTA-Ad. Overexpression of Dhx36 and Tial1 had no effect on V5-αENaC-Del5 mRNA expression. *P<0.05 by Kruskal-Wallis tests and Dunn's post-hoc tests compared experimental vectors to the empty vector; #P<0.05 by Mann-Whitney U-tests compared experimental vectors to the to complete 3'UTR mutant; n≥6 for each experimental condition. (C) RNA-binding proteins present in extracts of alveolar epithelial cells were purified by affinity chromatography using in vitro transcribed αENαC 3'UTR or deletion 5 3'UTR. The affinity-purified RBPs were subjected to SDS-PAGE for immunoblotting detection of Dhx36 and Tial1. Less enrichment of Tial1 and Dhx36 was detected with Del5 αENaC 3'UTR lacking the proximal region of αENaC 3'UTR compared to the whole sequence. Representative immunoblots for each RBP are presented. (C) The enrichment of Dhx36 and Tial1 determined by immunoblot analysis are depicted for each RBP. The data are expressed as relative enrichment ± SEM compared to Del 5. *P<0.05 by one-sample t-test compared to Del 5 (n=4 for each RBP).